
Elimination of Sobriety Requirements and 1115 Waiver Approvals Signal Progress Toward Equitable HCV Treatment Access
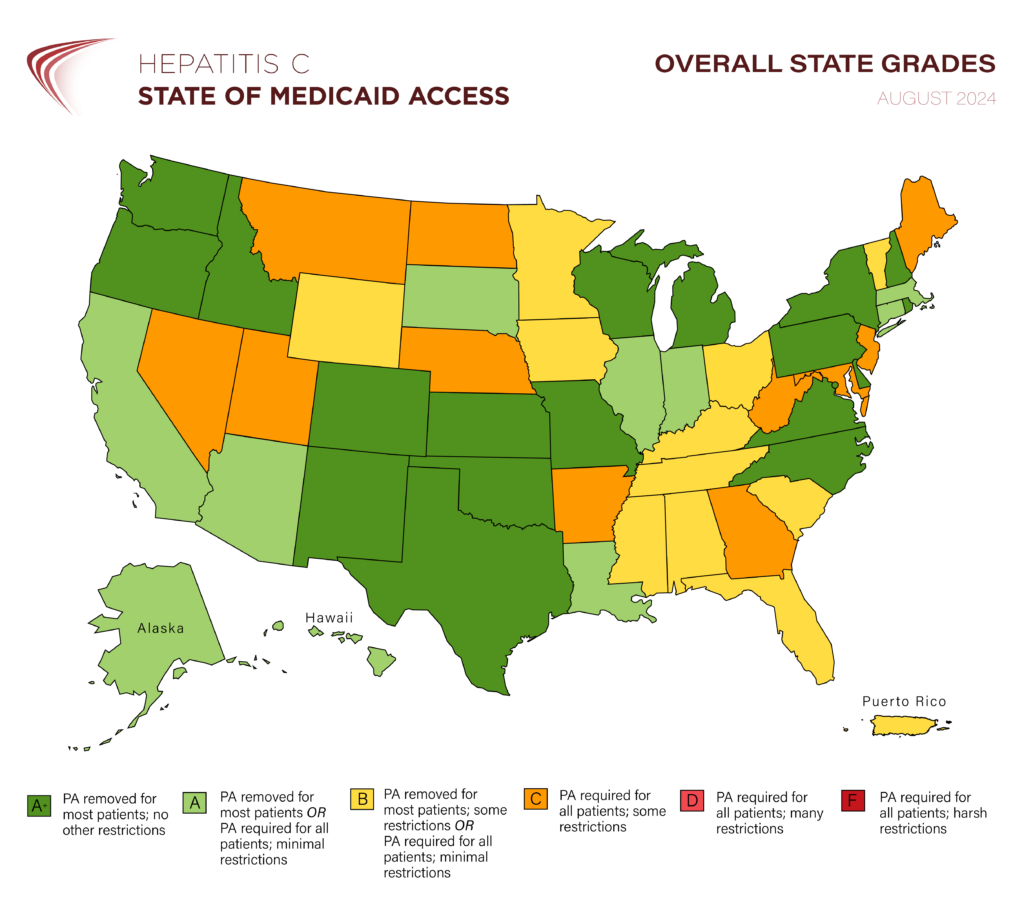
Washington, D.C. – August 14, 2024 – The Center for Health Law and Policy Innovation of Harvard Law School (CHLPI) and the National Viral Hepatitis Roundtable (NVHR) recently published updates to their state report cards reflecting changes to hepatitis C treatment access in Medicaid programs since the organizations’ February 2024 National Snapshot Report. Notably, Arkansas’ removal of substance use restrictions means there are no longer any jurisdictions that require sobriety as a pre-requisite to hepatitis C treatment.
“Significant progress has been made thanks to the unwavering advocacy of diverse stakeholders who believe that everyone deserves access to hepatitis C treatment,” said Adrienne Simmons, Director of Programs at NVHR. “But there is still work to be done to reach our goal of HCV elimination, and our collective voices are more important than ever. We must keep our foot on the pedal and push Congress to fund a national hepatitis C elimination program.”
In the last six months:
- 1 more state (NM) removed prior authorization requirements, becoming the 29th jurisdiction to do so.
- 2 states (AR, IA) removed substance use restrictions.
- 1 state (IA) removed prescriber restrictions.
- 1 state (IA) removed retreatment restrictions.
But perhaps even more striking are the federal publications on HCV elimination and 1115 waiver approvals that have emerged since our last report card update.
New data released by the Centers for Disease Control and Prevention (CDC) has underscored the critical need to eliminate unnecessary barriers to hepatitis C treatment, such as prior authorization requirements. The findings of this analysis suggest that the number of people with Medicaid who are treated for hepatitis C was lower in jurisdictions without Medicaid expansion and those with fibrosis and sobriety restrictions on HCV treatment. These restrictions have been shown to significantly impede access to care and delay treatment initiation. CHLPI and NVHR urge policymakers to prioritize policies that streamline access to life-saving HCV medications.
In addition, a recent report from the Congressional Budget Office (CBO) highlighted the potential cost-savings of expanding HCV treatment among Medicaid enrollees. The report found that increasing HCV treatment rates would result in substantial net savings to Medicaid due to reduced healthcare costs associated with liver complications, reinforcing the economic benefits of policies and programs that get more people treated and cured. CBO’s preliminary analysis, conducted to inform a fuller assessment of the budgetary impact of a proposed National Hepatitis C Elimination Program, should bolster state Medicaid programs’ confidence that removing barriers to HCV treatment is a fiscally sound policy.
Also promising, the Centers for Medicare & Medicaid Services (CMS) has approved waivers for an additional six states to implement pre-release Medicaid coverage for people leaving carceral facilities. By offering Medicaid coverage before release, the initiative aims to address this population’s elevated health needs, such as disparate rates of substance use disorder, mental health issues, and significant health-related social needs. The waivers also provide states with the discretion to address other conditions, and three approved states (UT, VT, and NM) have explicitly included HCV treatment in their design. CHLPI and NVHR encourage all states to similarly prioritize HCV care in their pre-release Medicaid programs.
“These recent developments are significant steps forward in the fight against hepatitis C,” said Elizabeth Kaplan, Director of Health Care Access at CHLPI. “It is important that states continue to work relentlessly to remove barriers to treatment for people living with HCV, incarcerated individuals, and those living with substance use disorders. The research supports what has long been apparent on the ground—that treatment restrictions create undue barriers to those seeking hepatitis treatment. It is now also clear that a national HCV elimination effort would yield significant cost savings for the government. We have the potential to make remarkable progress towards HCV elimination.”
CHLPI and NVHR remain committed to working with policymakers, healthcare providers, and community organizations to eliminate hepatitis C in the United States.
About The Center for Health Law and Policy Innovation of Harvard Law School
The Center for Health Law and Policy Innovation of Harvard Law School (CHLPI) advocates for legal, regulatory, and policy reforms to improve the health of marginalized populations, with a focus on the needs of low-income people living with chronic illnesses and disabilities. CHLPI works to expand access to high-quality health care; to reduce health disparities; to develop community advocacy capacity; and to promote more equitable and effective health care systems. CHLPI is a clinical teaching program of Harvard Law School and mentors students to become skilled, innovative, and thoughtful practitioners as well as leaders in health and public health law and policy. For more information, visit www.chlpi.org.
About the National Viral Hepatitis Roundtable (NVHR)
An initiative of HEP, the National Viral Hepatitis Roundtable is the largest network of patients, providers, public health leaders, and community partners breaking down barriers to care across the United States. For over 20 years, NVHR has driven progress toward viral hepatitis elimination through knowledge sharing, advocacy, and policy change. For more information, visit www.nvhr.org.
MEDIA CONTACTS
Jenni Todd: (206) 934-0711
jennit@hep.org
Ada Ezeokoli: (617) 384-9618
aezeokoli@law.harvard.edu
