February 29, 2024
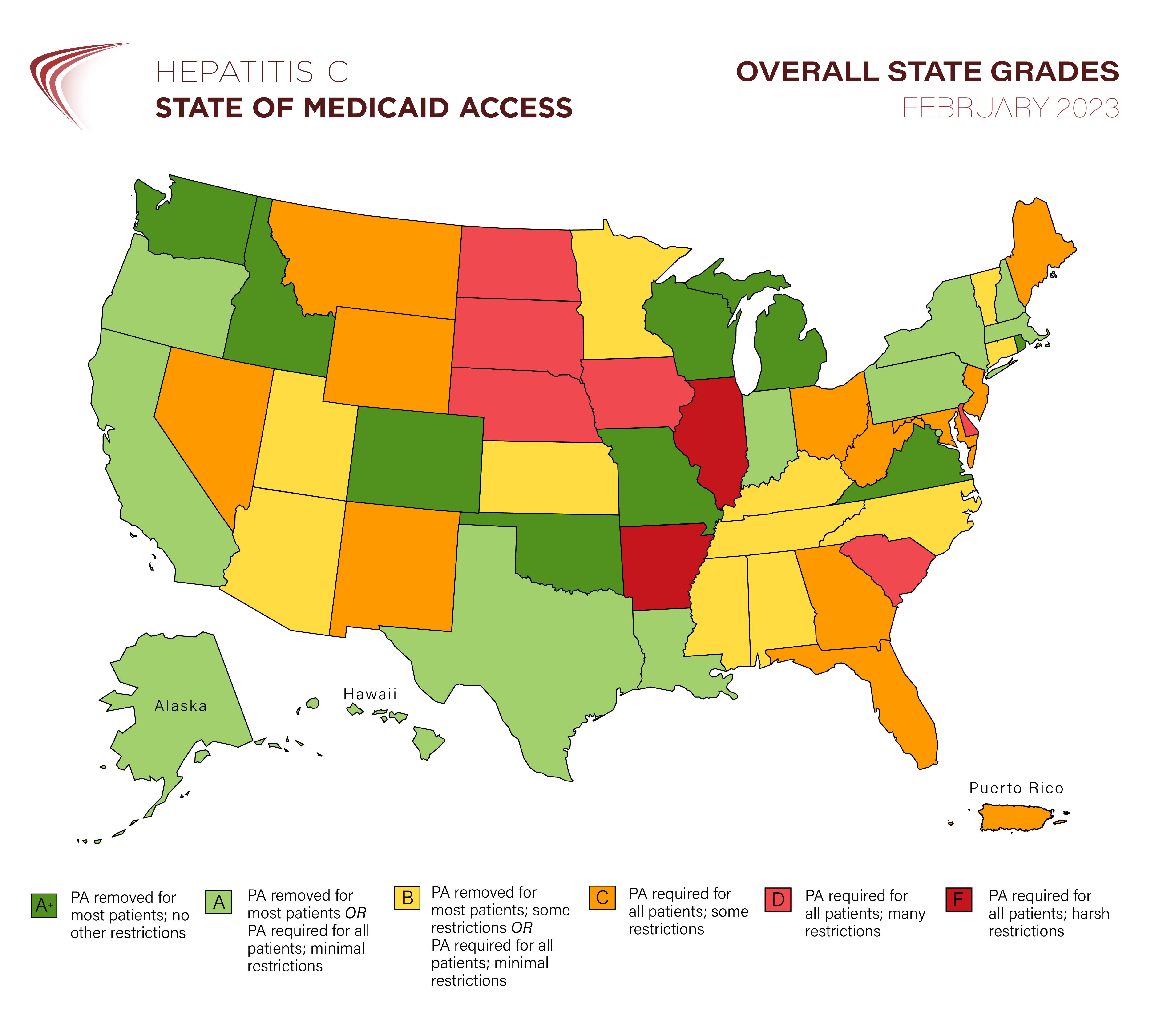
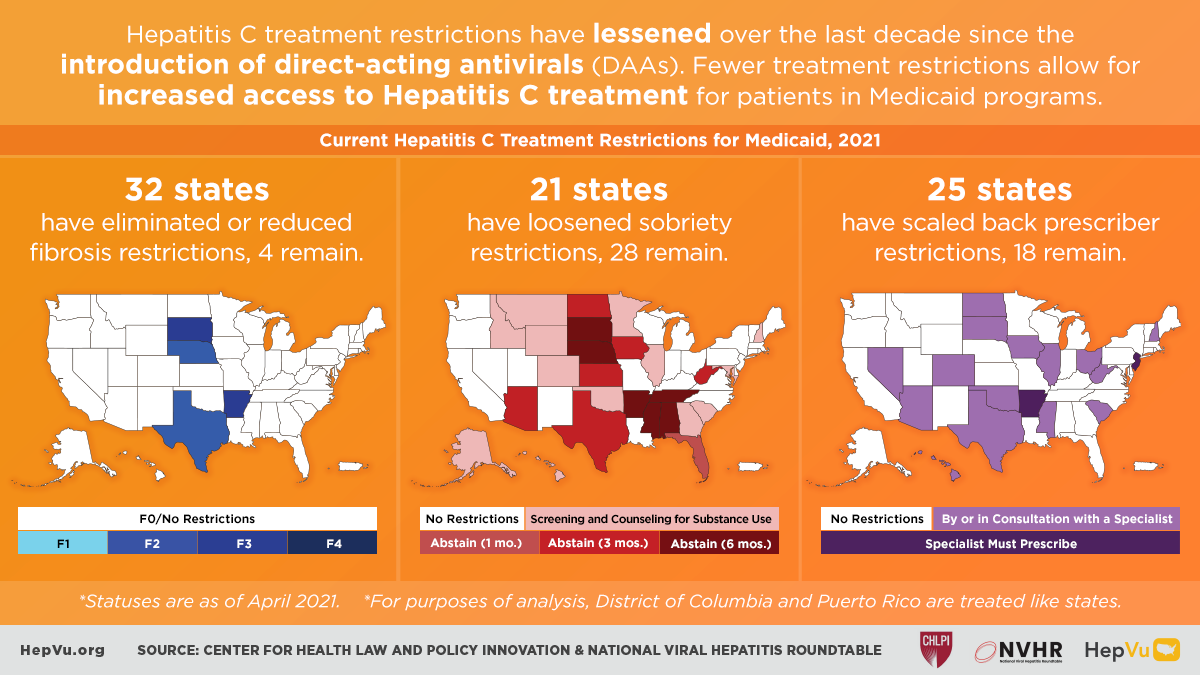
It’s official: more than half of state Medicaid programs no longer require prior authorization for first-time hepatitis C treatment
Newly updated for 2024, state report cards herald the end of fibrosis restrictions while warning about needs for parity with managed care organizations and restrictive retreatment policies FOR IMMEDIATE RELEASE … [Read More]