What are Managed Care Organizations and how do they affect Medicaid access?
August 28, 2023 | Category: No categories
August 28, 2023 | Category: No categories
Managed Care Organizations, or MCOs, are private companies that contract with state Medicaid programs to administer benefits to people who are enrolled in the state’s Medicaid plan.
Nationwide, 72% of all Medicaid beneficiaries receive their health coverage through an MCO, and all but 10 state Medicaid programs contract with at least one MCO as of August 2023.
As such, these contracted organizations and how they individually operate majorly impact the majority of Medicaid recipients’ access to care like hepatitis C treatment.
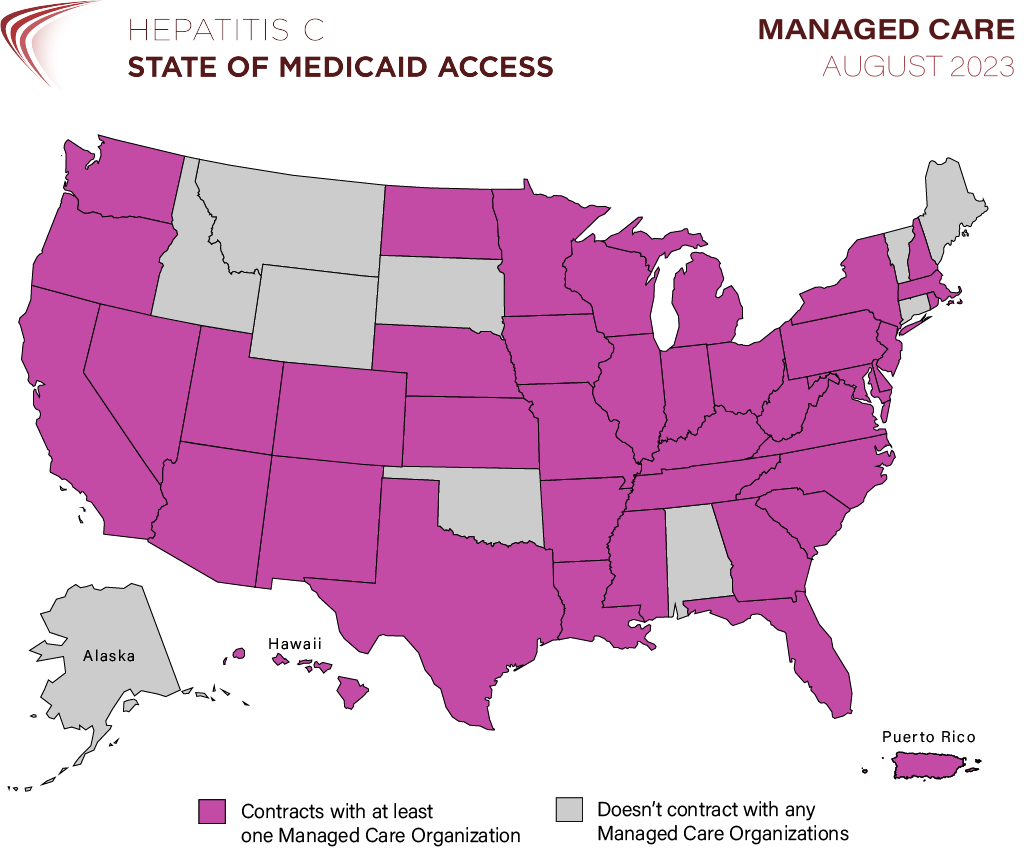
Hepatitis C: State of Medicaid Access tracks and documents hepatitis C (“HCV”) treatment access policies in state Medicaid programs across the country. For states that do contract with MCOs, we track whether the state’s official Medicaid policy (known as the “fee-for-service” or “FFS policy”) is in parity with, or equivalent to, any MCO policies.
Federal regulations require MCOs to provide services in the same “amount, duration, and scope” as would be provided under the state’s official FFS plan. See 42 CFR § 438.210. Additionally, MCOs may not “arbitrarily deny or reduce the amount, duration or scope of a required service solely because of diagnosis, type of illness, or condition of the beneficiary.” Id. In 2015, CMS issued guidance clarifying that these regulations apply to DAA treatment specifically, and noted: “While managed care plans may place appropriate limits on DAA HCV drugs using criteria applied under the state plan, such as medical necessity, the managed care plan may not use a standard for determining medical necessity that is more restrictive than is used in the state plan.” Id. MCOs may use different “utilization controls” than FFS, but CMS has cautioned that any such restrictions should be “carefully monitor[ed] . . . to ensure that [MCOs] are providing appropriate access to covered services and benefits.” Id.
Policies and processes that vary by MCO create access disparities within a state’s Medicaid program and cause confusion among providers and patients. As of August 2023, 14 states (27%) continue to have MCO parity issues: 11 states (21%) contract with at least one MCO that publishes a more restrictive policy than FFS, and 7 states (13%) have at least one MCO that does not publish its criteria at all. Shockingly, these problems persist even in states whose FFS policies have removed prior authorization entirely for most patients. A total of 25 jurisdictions have now removed prior authorization in their Medicaid programs, yet of those, 7 still have at least one MCO with a more restrictive or unknown policy: Arizona, DC, Florida, Hawaii, New Hampshire, Oregon, and Texas.
@2025 HEPATITIS C: STATE OF MEDICAID ACCESS
As of February 2024, 28 states have removed prior authorization for first-time treatment: AK, AZ, CA, CO, CT, DC, DE, FL, HI, ID, IL, IN, KS, LA, MA, MI, MO, NC, NH, NY, OK, OR, PA, RI, TX, VA, WA, WI.